

Introducing novel serological markers for inflammatory bowel disease (IBD) which improve sensitivity and specificity to aid in differential diagnosis and provide valuable prognostic information about disease behavior.
As of 2016, the IBDX panel biomarkers: gASCA, ALCA, ACCA, AMCA, Anti-L and Anti-C were
analyzed on over 7500
of patient samples in multiple independent clinical studies around the globe.
For publications see >>How to
order
Multiple studies have shown when the IBDX biomarkers are combined with pANCA higher sensitivity and sensitivity is obtained. The IBDX achieved 0.81 to 0.846 area under the curve (AUC) for diagnosing IBD vs non IBD 2, 4, 6, 9, 13, 18 including in pediatric population.14

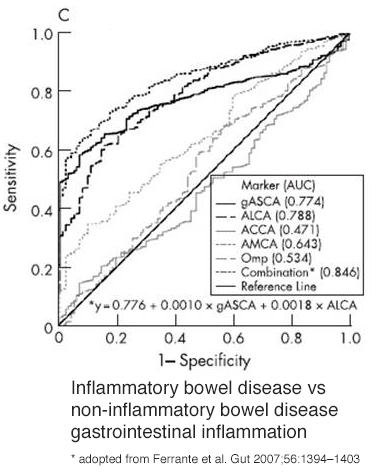
*adopted from adopted from: Seow et. al. AJG 2009; 104(6): 1426-1434
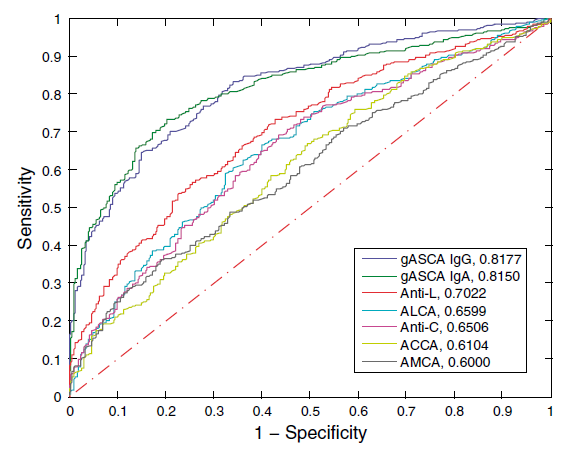
*adopted from Dotan et. al. Gastroentrology 2006, 131:366–378
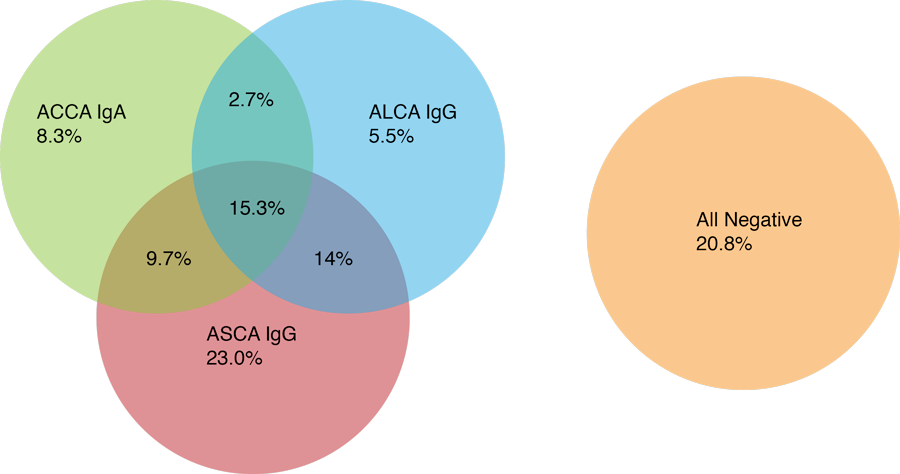
Multiple studies have shown that the IBDX panel identifies 35% and 56.4% of ASCA negative patients 1, 2, 3, 4, 6, 8, 9, 12, 13, 18 including pediatric population14, 16
Inflammatory Activity and Progression of Bowel Damage in a Theoretical Patient with CD.
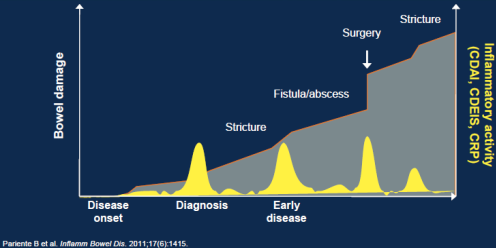
Studies have shown correlation between the number of positive serological IBDX markers and Crohn's Disease behavior and abdominal surgery1, 2, 3, 6, 9, 13, 18 including pediatric population 14, 16
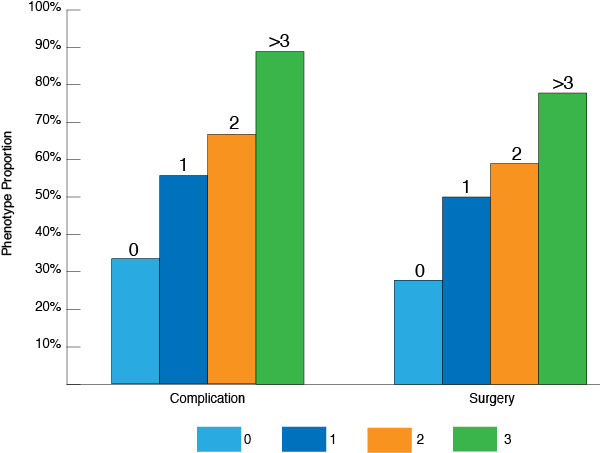
number of positive serological markers
*adopted from Reider et. al. 2010 IBD 16 (2) 263-274
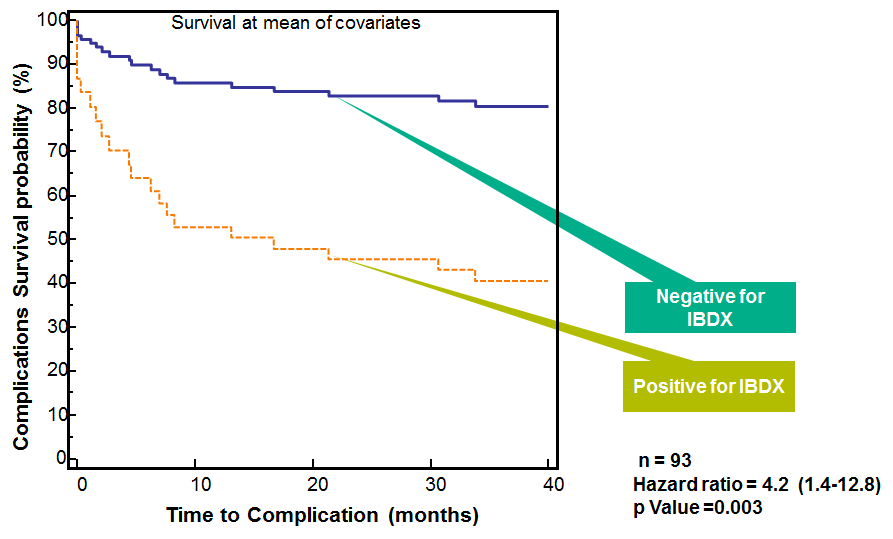
*adopted from Rieder et al., Inflamm Bowel Dis 2010 16 (8), 1367-1375.
Prospective study demonstrated that Chron’s disease patients positive for at least two IBDX antibodies are at a higher risk to progress faster10
Studies have shown that the IBDX ACCA, AMCA and Anti-L can predict Ulcerative Colitis patients who went through ilial pouch-anal anastomis (IPAA) surgery and developed pouchitis 5, 7, 17
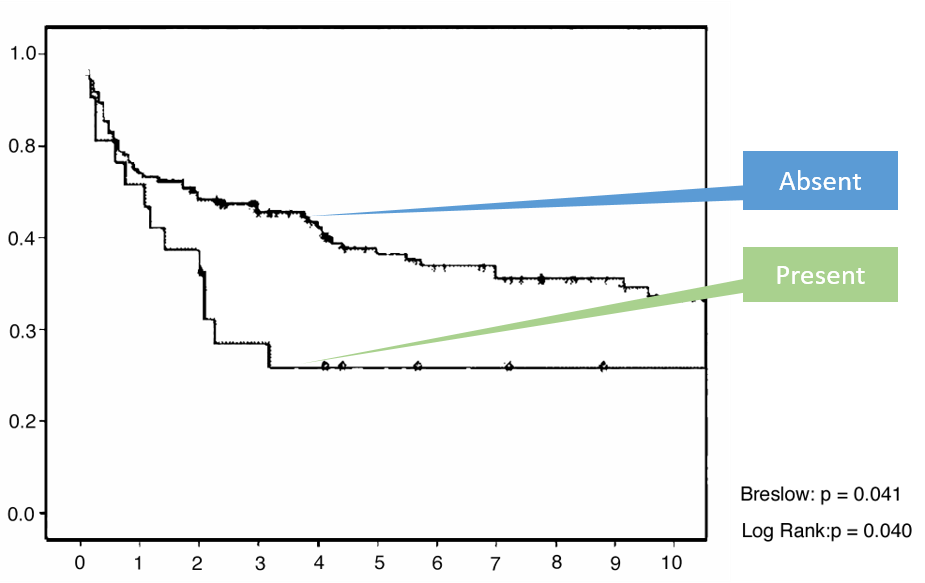

* adopted from Ferrante et al., JCC 2008 (2), 142-151.
If the IBDX panel is not available at your local lab, Glycominds via its affiliate Pan Laboratories,
a U.S. CLIA certified lab provides the IBDX testing international services. Using our proprietary dry serum
technology a regular mail shipment at a cost effective price is available.